Air pressure
The air is surrounding the Earth, forming the atmosphere (see picture on the right).
On each square metre
of the surface of the Earth (approx. 5·10⁸ km² and/or 5·10¹⁴ m²) the air column weights about 10 tons; this is the
atmospheric pressure.
The pressure (weight of the atmosphere per surface unit) at sea level is about 101'000
N/m², corresponding to approximately 101'000 Pa or 1010 hPa. The average atmospheric pressure at sea level (0 m asl)
is normed at 101'325 Pa = 101.325 kPa = 1'013.25 hPa = 1.01325 bar. Bar is the more ancient unit for measurement of
the atmoshperic pressure, which was originally considered to be 1 bar (1'000 hPa), until modern and more precise
measurements showed the average on Earth to be 1'013.25 hPa.
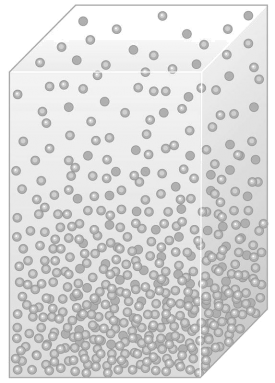
The air pressure decreases with altitude.
It is easy to imagine that the air at the bottom, close to the ground, is compressed by the air
molecules above it and therefore more dense (see picture on the left).
In the upper layers, the pressure is
lower and decreases with the altitude. Since the air pressure always decreases with the altitude and is never equal
at two different altitudes, one can also imagine the atmosphere as a pile of layers with equivalent air pressure
(isobar layers).

In the picture on the right-hand side, such levels are represented by the printing pressure p0, p1, p2…. p6.
Isobar means lines of "same barometric" pressure.
Source: E. Parlow (2008).




